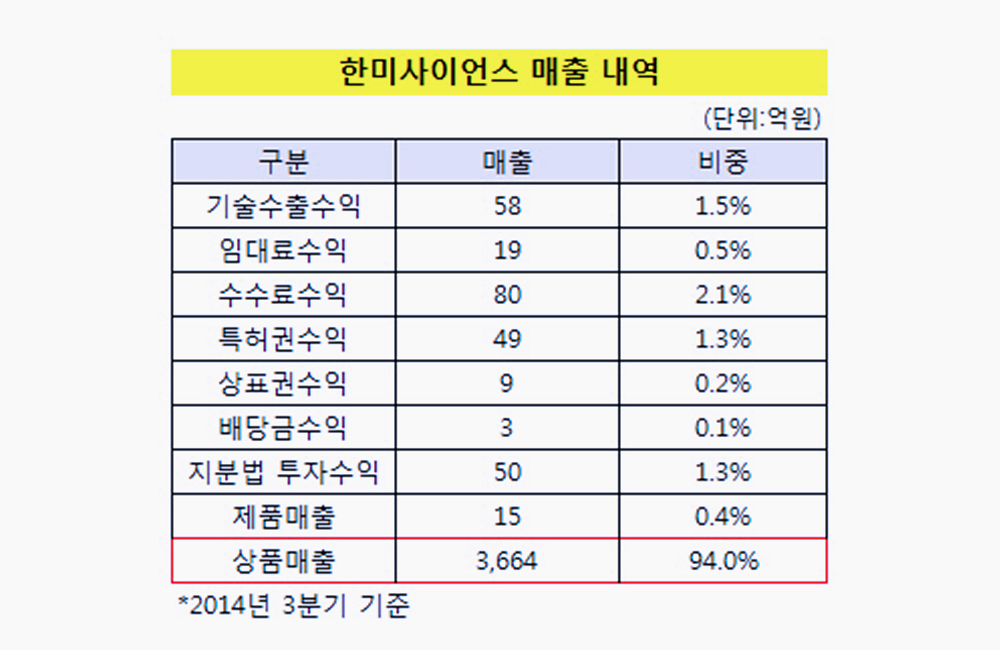
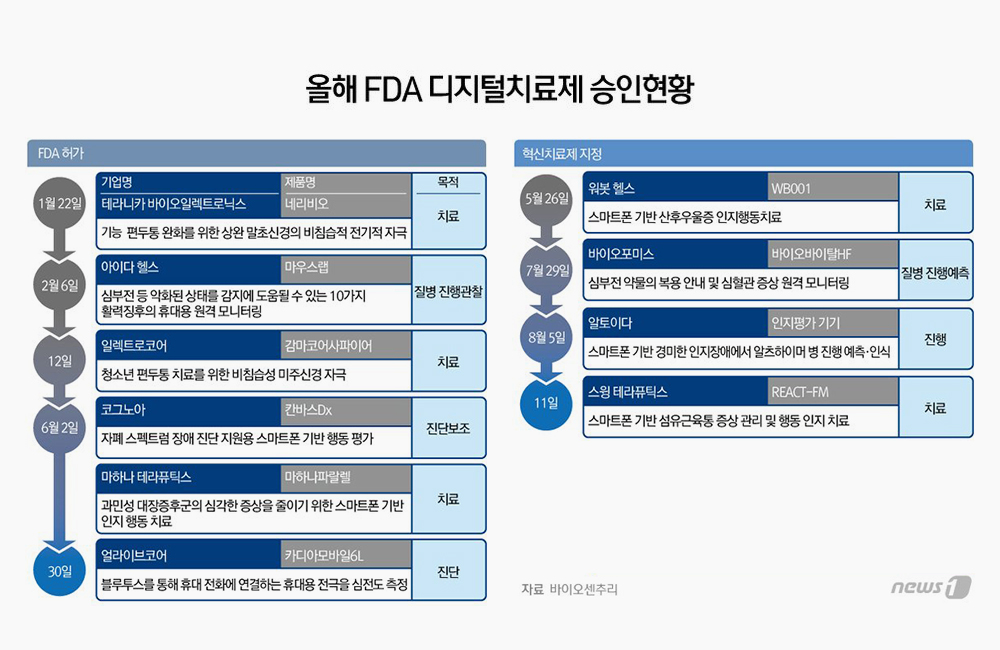
Korean
medical AI solution developers said the Korean government set excessively high
standards for new health technology assessment, a step required for getting
health insurance benefits.
Korean physicians are highly interested in AI-backed medical practice, and companies are actively developing AI healthcare solutions.
However,
not a single firm has passed the new health technology assessment so far, let
alone health insurance coverage.
The
National Evidence-based Healthcare Collaborating Agency (NECA) introduced the
new health technology assessment in 2007 to evaluate health technologies' safety
and clinical usefulness.
Most
companies working on new medical technologies aim to pass the NECA’s technology
assessment because it is almost a prerequisite for winning reimbursement.
However,
NECA has not placed any medical AI product in the new health technology
category to date.
The
agency even classified “an innovative medical device” named by the Ministry of
Food and Drug Safety in the existing technology category.
Industry
officials criticized NECA for failing to meet the purpose of the new health
technology assessment and hampering Korea’s technology power in medical AI and
the market’s vitality.
They
said it would be very difficult for doctors to use non-reimbursable medical AI
solutions.
Most
AI-backed medical devices are being developed as in vitro diagnostic products.
Unless they get insurance benefits, medical institutions have little reason to
choose AI-using medical devices, they said.
If
NECA classifies a new medical device in the existing technology category, the
developer can commercialize it immediately.
However,
clinicians’ demand for the new product with the existing technology is not
high.
So,
if a medical AI product is classified in the existing technology, the developer
can no longer sell the technology in the market.
The
purpose of the new medical technology evaluation system is to evaluate new
types of technologies that require safety and effectiveness evaluation and to
classify technologies that are completely identical to the existing ones, which
can be commercialized well as existing technologies, an industry official said.
“In reality, many
medical AI technologies are facing an ‘evaluation hurdle’ as the agency keeps
classifying them as existing technologies.”
“For the
commercialization of innovative medical technologies and industrial
advancement, we need insurance benefits that are different from existing ones,”
he emphasized.
Another
industry official said NECA should be more flexible about the technology
assessment and ease standards.
He
said it was understandable that the regulator could make a conservative
judgment on medical technologies because they directly affect the health of the
Korean people.
Still,
the new health technology assessment does not consider the reality of companies
and the development environment, he said.
For
example, one of the NECA’s standards demands a company state the product is
indicated for which disease or surgery, he said.
“Medical AI products
can be used broadly. If we state all of them, we have to spend too much time
and costs to verify the safety and efficacy. But if we narrow them down, the
product can be used for only about 1,000 cases per year, which is not
profitable at all,” he said.
NECA
said it implemented the new health technology assessment program “to prevent
indiscriminate use of unverified medical technologies and to protect the
public’s right to health.”