Digital therapeutics (DTx) is a new
category of medicine that refers to treatments delivered directly to patients
via software or apps. The scope of DTx encompasses treatment, management, and
prevention of a broad spectrum of diseases and disorders. As such, DTx are part
of a larger ecosystem of digital health technologies that provides support for
patients, clinicians, and medical institutions. These technologies include
patient- and clinician-platforms, as well as the unseen operational components.
DTx differs from the multitude of mental
health apps on the market because they are meant to be prescribed or
recommended by a health care professional; part of a comprehensive treatment
plan; evidence-based; and subject to the same regulatory requirements as
traditional medical treatments. The development of DTx is an area that affects
patients, clinicians, payors, and policymakers. The Digital Health Alliance1
has put forth a 10-point list of core principles for DTx products as a first
step in operationalizing the industry; these principles also help to
distinguish DTx products from unregulated apps.
In order to use DTx effectively, we must
understand its relationship to the following categories: patients, clinicians,
health care systems, and innovation/discovery, especially as it relates to
comorbid disorders.
DTx and Patients
As many as 80% of adults in the US have a
smartphone, and many of them spend hours per day interacting with their
devices.2 Although most of that time is likely for entertainment purposes,
there are also opportunities to improve their health.
Recent advances in technology have led to
the unobtrusive, seamless collection of patient-generated health data from
smartphones, which can be used to improve patient care and outcomes. For
example, smartphones can collect data on sleep, activity, physiology, and
device use, as well as provide an environmental context. Digital phenotyping
refers to the “moment-by-moment quantification of the individual-level human
phenotype in situ, using data from smartphones and other personal devices,” and
DTx takes it to the next level by recommending actions based on that
phenotyping.1
The near ubiquity of these devices means
that the clinical applications of digital phenotyping and DTx are highly
scalable; their use also allows the individuals’ data to be used as their own
unique baseline. Research has demonstrated the feasibility and acceptability of
physiological data capture for various disorders.3 The hope is that digital
markers of behavioral change (ie, observable changes in sleep, physical
activity, and social interaction) might become sensitive measures of meaningful
variation in functional status, symptoms, and risk for adverse outcomes in
patients, thereby better guiding and personalizing care.3,4
Acceptability of app use, and DTx in
general, is an encouraging sign. Scalability will likely depend on age and
generational influences (younger individuals may potentially be more amenable
to DTx use), comfort with technology in general, and socioeconomic disparities
(ie, more affluent individuals with better phones/wearables will be able to
access more nuanced DTx options).
A yet unexplored problem is adherence to
DTx use once prescribed; as large-scale implementation becomes a reality, this
issue will need to be appropriately addressed. “App burnout,” a phenomenon
referring to the short-term use of apps, may be relevant to DTx as the
prescribed length of time (ie, weeks, months, years) increases. Current studies
in mental health, for instance, have looked at DTx with contingency management
and cognitive behavioral therapy for 12 to 16 weeks; medication active sensing
systems meant to improve adherence, such as Abilify MyCite (aripiprazole
tablets with sensor), have been helpful in short-term studies and have been
evaluated for a maximum of 26 weeks per the United States Food and Drug
Administration (FDA) approval. Thus, although the short-term implementation of
DTx has been an attractive option for the management of chronic disorders, much
is still unknown about the interaction of DTx with the naturalistic course of a
chronic illness, and much remains unknown about the best times to implement DTX
measures (Figure).
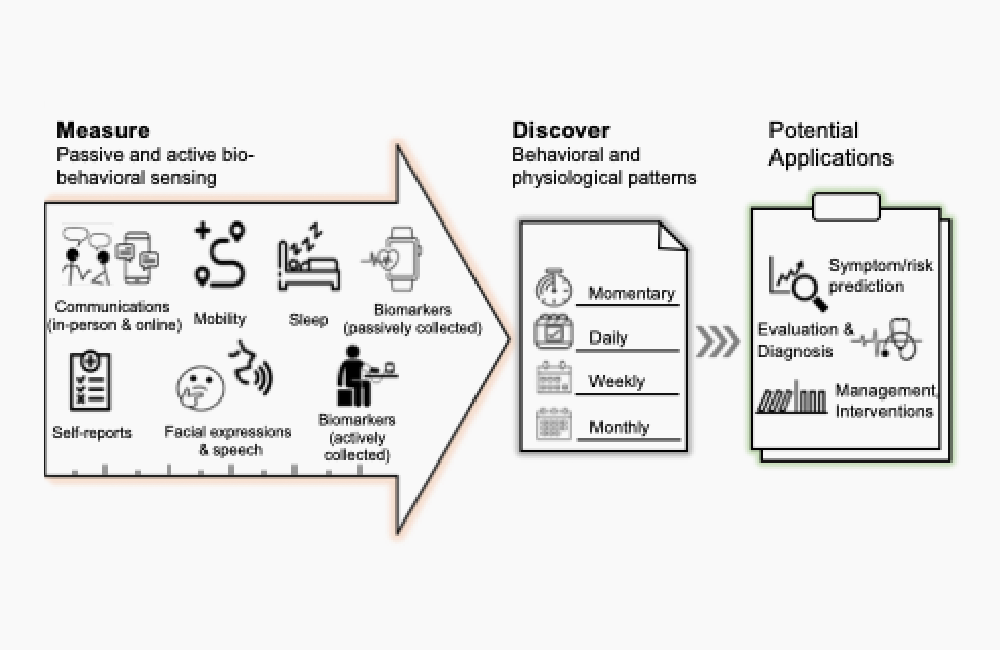
Figure. Extracting Biobehavioral Markers From Sensors for the Development
of DTX
Clinician Perspectives
DTx have been developed over the past 15 to
20 years to target chronic medical and mental issues, with the assumption that
many of these conditions can be improved by shaping behaviors. However,
physicians adopting DTx in chronic management face ethical, legal, and
practical challenges. In the case of psychotic disorders, ingestible sensors
(despite positive data in promoting adherence) are seen as potentially
decompensating factors in a patient’s delusion, thus limiting adoptability.5,6
Although not strictly DTx, deep brain stimulation
devices have faced adoptability challenges for depressive and psychotic
disorders because of clinician concerns that they may worsen the primary
disease.7 Gartner Inc described the concept of the hype cycle, a maturity
technology timeline that relates technology visibility to time. This concept
explains the issues involved in DTx adoptability, including novelty of the new
modality, expected value, expectation inflation of opinion related to
innovation, and time needed for the technology itself to reach maturity.8
Clinicians theoretically support the idea
of closing treatment gaps and would likely be open to leveraging concrete
treatment planning and adherence tools to reinforce what was discussed
in-session. However, despite FDA approval of DTx apps (such as Welldoc’s
BlueStar for Type II diabetes management and Pear Therapeutics’ reSET for the
treatment of substance use disorder), DTx is still globally an underprescribed
and underused modality. In addition, the adoption of DTx in clinical practice has
been slow.9 Why is this the case?
From a clinician perspective, there are a
number of hurdles to overcome, including lack of familiarity with DTx, lack of
time to introduce and administer DTx, and the lack of DTx integration with
electronic health records. Similarly, there are obstacles in billing for DTx.
Although they exist, very few physicians are employed in systems that offer
digital formularies. Thus, adoption by physicians is an unsolved challenge for
DTx implementation. In order to achieve DTx scalability, it is necessary to
change the clinical visit microenvironment and make DTx a viable treatment
decision.
Systemic and Regulatory Perspectives
Reimbursement and oversight are the 2 big
areas of concern in health care systems, yet the regulatory landscape
surrounding DTx is still in flux.10 The first question that must be answered is
who is responsible for regulating DTx. The second is, what is the relation of
DTx to machine learning and whether changes in software or updated machine
learning algorithms would require reapproval or new approvals. The Total
Product Lifecycle approach currently under consideration by the FDA could
address some of these concerns.
Currently, approved or cleared products
have received their acceptance from the FDA Center for Devices and Radiological
Health, some using the Breakthrough Therapy designation. The aim, ideally,
would be a path for software-as-a-medical-device (SaMD) products, with
predetermined steps through the lifecycle of the software. This will require
modernization of the FDA paths for medical devices, which were written in
1976.10
System-level challenges of DTx also include
cybersecurity. DTx interfaces with, and is reliant on, multiple nonmedical
entities, including internet, phone, and cloud storage service providers. There
are currently no global answers to these issues, but patients and clinicians
alike will be reluctant to transmit sensitive health data over unsecured
channels. Moreover, reimbursement for DTx is moving slowly but surely. Some
private insurers pay for DTx as prescribed, but nationwide reimbursement codes
remain uncommon.
The Power of Innovation
Thus far, the main DTx developments have
been the delivery of already proven treatments via electronic/software means.
However, the future of DTx will likely include higher-order constructs and
products that address comorbid disorders. Digital innovation in mental health
already exists, and is accelerating rapidly, which is a good thing. Digital
innovation serves 3 broad purposes.
First, digital innovation can increase
access to mental health care. With the currently available technologies, there
has been a continual improvement in the delivery of mental health services via
telemedicine/telepsychiatry. Telepsychiatry has been important in facilitating
patient follow-up by reducing travel, thereby helping longitudinal engagement.
This, in turn, has improved adherence and reduces loneliness and
misinformation.11 Although telepsychiatry may not always be optimal for patient
engagement and patient-physician rapport, studies have shown it is not inferior
to face-to-face care.11 Feasibility and success in delivering therapeutic
interventions, such as cognitive behavioral or supportive therapy, and sleep
hygiene education via the internet/phone/apps have been demonstrated.12-14
Second, digital innovations provide a new
arsenal of tools to measure aspects of patient biology and behavior in ways
that were not previously possible. This objective data collection, in turn,
will likely allow better diagnosis and will allow clinicians to adjust care, as
necessary.
Third, the fast-expanding repertoire of
artificial intelligence tools enables the discovery of complex relationships
between new forms of data and mental health conditions. As a result, novel
ideas are emerging to quantify complex concepts like loneliness, mood swings,
and cravings/binge eating that may underlie worsening mental health symptoms.
Future DTx developments are expected to go beyond already proven treatments.
Mobile and Wearable Technologies in
Mental Health
Seppälä et al15 reviewed mobile phone/wearable
sensor–based mobile studies (N = 33) for mental health conditions between 2009
and 2018. The majority of the studies targeted unipolar depression or anxiety
in healthy participants, with fewer studies focusing on bipolar and psychotic
disorders. The Global Positioning System (GPS) was the most common sensor used.
Features such as reduced mobility patterns and time spent at home during
specific time intervals of the day were associated with scores indicative of
depression or anxiety, as measured by questionnaires such as the Patient Health
Questionnaire (PHQ)-9, Generalized Anxiety Disorder (GAD)-7, or by Ecological
Momentary Assessment (EMA) self-reported mood.15,16 The second most common
assessment was physical activity, measured with an accelerometer/gyroscope;
this was followed by phone call logs.16
Higher-level clinically relevant
constructs, such as behavioral regularity, may be useful to better understand
behavior patterns. Data from wearable and mobile phone sensors showed that 2
behavioral regularity indices17 were correlated with perceived stress scale
scores, the mental component scale of the 12-Item Short Form Health Survey
(SF-12), and daily self-reported mood.17 The indices included a sleep
regularity index that quantified the regularity of sleep time, wake time, and
sleep duration, and a daily mobility index that detailed routines based on GPS
data. Using behavioral rhythm markers (eg, ultradian, circadian, and infradian
rhythms) estimated from mobile phone sensors, these data predicted self-reported
symptoms from individuals with schizophrenia (the machine learning algorithm is
able to predict when patients will be reporting certain symptoms based on
behavioral patterns collected as above).18,19 Changes in daily behavioral
patterns based on mobile phone sensors were used to predict symptom resurgence
in patients with schizophrenia.19
The higher-level construct of social
ambiance can likewise be obtained by unconstrained day-long recordings from
wrist-worn audio-bands that estimate the number of simultaneous speakers.20 The
number of simultaneous speakers was used as a proxy for an individual’s
sociability, as social isolation is often a symptom of mental illness. By
classifying audio into 4 levels (ie, quiet, low, mid, and high social ambiance),
researchers found that individuals with depression or psychosis spent less time
in diverse environments with higher social ambiance levels. Moreover, social
ambiance patterns are associated with the severity of self-reported depression
and anxiety symptoms, and personality traits such as neuroticism and
agreeableness.
Despite strong preliminary evidence
associating sensor data with mental health conditions, many challenges lie
ahead in data-driven modeling and inference. This issue is a major challenge
associated with the scalability of DTx development, as it requires industry and
academic collaborations. For instance, computational models need to learn
associations between individual patients’ biomarkers and mental health
constructs (eg, symptoms, critical events). Every patient has different
behavioral patterns and physiological responses, so the challenge becomes, how
to determine regular/irregular patterns for each patient, or each group of
patients.
A single computational model might not be
able to fit the heterogeneity of different patients. Similarly, patients’
symptoms are based on their self-reports, which are subject to variabilities
and biased perceptions. Current technology has a limited ability to detect
biased self-reports, thereby limiting the usability of deep learning. Deep
learning is a popular and powerful approach to developing complex neural
network models; however, it is hard to interpret why certain models work well,
and model-building requires a significant amount of data per patient.
Comorbid Disorders and Paradigm Shifts
Although the overlap between depressive
disorders and diabetes is not well understood, it has been hypothesized to be
potentially linked to immune system overactivity/systemic inflammation,
psychoneuroendocrine dysregulation, cognitive functioning, and common genetic
risk factors.4,11,21,22 This type of comorbidity could be effectively targeted
with DTx if models are built to understand this disease overlap,23 especially
as the field undergoes a paradigm shift.
One of the biggest conceptual ongoing
shifts in psychiatry is characterizing mental illnesses dimensionally, in
contrast to the current practice of categorical classification. Taking major
depressive disorder (MDD) as an example, a shift in both diagnosis and
management can be achieved by leveraging technology and by adopting a more
modern, innovative approach to diagnosis. In the current diagnostic paradigm,
MDD is defined in the DSM-5 as having 5 of 9 symptoms (with necessary inclusion
of depressed mood and/or anhedonia). Thus, it is possible that 2 patients with
the same MDD diagnosis have no overlapping symptoms: one has depressed mood,
the other anhedonia; one eats too much, one eats too little; one sleeps too
much, the other too little; one is suicidal, the other is not, etc.
The National Institute of Mental Health has
established the Research Domain Criteria (RDoC) program, which recommends that
mental health research be focused on symptoms instead of diagnoses, and studies
should be done not categorically, but dimensionally, in a manner that allows
overlap with tech-based objective information gathering. The RDoC highlights
the following neurobiological transdiagnostic research dimensions: negative
valence, positive valence, cognitive processes, systems for social processes,
and arousal/regulatory systems. The current state of DTx is
patient-centered/patient-driven information collection, but most DTx
structures, thus far, do not fit categorical or dimensional psychiatric
diagnostic frameworks; instead, they focus on individual symptoms and may be
the simplest level of dimensional classification.
In the dimensional paradigm, a symptom like
low energy can be translated into low physical activity/low mobility/low
socialization by sensor detection. Those, in turn, can be addressed with a
focused intervention, such as behavioral activation. This approach has
measurable outcomes, is easy to explain to patients, and is more amenable to
process improvement based on real-time feedback. The current approach to MDD treatment,
which usually starts with the addition of a selective serotonin reuptake
inhibitor (SSRI), aims to improve feelings of depression/anhedonia generating
the low activity/low energy.
Going back to the issue of comorbidity,
taking an SSRI provides some improvement in survival in the context of
diabetes, but not the expected protective effect that is hoped for in diabetes
and depression.24,25 Intuitively, this makes sense as a lifting mood may not
fully translate into the desired energy increase, unless the patient
specifically learns the needed skills to effect this change.
The field has turned to dimensions that may
impact behavioral change, and those in turn will need to be measured and
relayed back to the patient in a feedback loop. A good illustration of this is
self-efficacy, or the belief that one has control over health behaviors.
Self-efficacy tends to be diminished in patients with chronic illnesses, who
may accept many symptoms as an inevitable part of the disease process, and lack
the understanding/ability to take control of their biological processes. Poor
self-efficacy may overlap with the social determinants of health and health
care disparities but, in general, studies on self-efficacy and specific
diabetes-related mitigating behaviors are promising.26-28 Taking care of
oneself would help to improve mobility/energy and, by extension, mood. At this
point, this stepped process lacks clarity and established protocols, but it
could be an excellent DTx goal for further development.
Concluding Thoughts
Leveraging DTx to improve mental and
physical health is likely to be the biggest, paradigm-shifting change that
medicine has known since the invention of antibiotics. However, how to apply
these tools remains an area that needs operationalization. DTx is at the nexus
of digital innovation and scalability/wide-scale use of digital interventions,
with more exciting developments on the horizon.
Dr Moukaddam is associate professor,
Menninger Department of Psychiatry & Behavioral Sciences, Baylor College of
Medicine, and Ben Taub Adult Outpatient Services Director, Medical Director,
Stabilization, Treatment & Rehabilitation (STAR) Program for Psychosis. Dr
Sano is an Assistant Professor at Rice University, Department of Electrical and
Computer Engineering, Computer Science, and Bioengineering. She also directs
the Computational Wellbeing Group. Dr Salas is an associate professor of
Psychiatry Research at Baylor College of Medicine. Dr Sabharwal is chair of the
Department of Electrical and Computer Engineering and the Earnest Dell Butcher
professor of engineering at Rice University.
References
1. Digital Therapeutics Alliance. Transforming Global
Healthcare by Advancing Digital Therapeutics. Accessed June 14, 2021.
https://dtxalliance.org
2. Statista. How much time on average do you spend on
your phone on a daily basis? Accessed June 22, 2021.
https://www.statista.com/statistics/1224510/time-spent-per-day-on-smartphone-us/
3. Brannon EE, Cushing CC, Crick CJ, Mitchell TB. The
promise of wearable sensors and ecological momentary assessment measures for
dynamical systems modeling in adolescents: a feasibility and acceptability
study. Transl Behav Med. 2016;6(4):558-565.
4. Kazukauskiene N, Podlipskyte A, Varoneckas G,
Mickuviene N. Insulin resistance in association with thyroid function,
psychoemotional state, and cardiovascular risk factors. Int J Environ Res
Public Health. 2021;18(7):3388.
5. Gerke S, Babic B, Evgeniou T, Cohen IG. The need for
a system view to regulate artificial intelligence/machine learning-based
software as medical device. NPJ Digit Med. 2020;3:53.
6. Dufort A, Zipursky RB. Understanding and managing
treatment adherence in schizophrenia. Clin Schizophr Relat Psychoses. Published
online January 3, 2019.
7. Gardner J. A history of deep brain stimulation:
technological innovation and the role of clinical assessment tools. Soc Stud
Sci. 2013;43(5):707-728.
8. Dedehayir O, Steinert M. The hype cycle model: a
review and future directions. Technol Forecast Soc Change. 2016;108:28-41.
9. Gordon WJ, Landman A, Zhang H, Bates DW. Beyond
validation: getting health apps into clinical practice. NPJ Digit Med.
2020;3(1):14.
10. Patel NA, Butte AJ. Characteristics and challenges
of the clinical pipeline of digital therapeutics. NPJ Digit Med. 2020;3(1):159.
11. Batastini AB, Paprzycki P, Jones ACT, MacLean N.
Are videoconferenced mental and behavioral health services just as good as
in-person? A meta-analysis of a fast-growing practice. Clin Psychol Rev.
2021;83:101944.
12. Barnes DM, Jarlais DD. Feasibility of a simple and
scalable cognitive-behavioral intervention to treat problem substance use. J
Subst Use. 2019;24(6):693-695.
13. Erten Uyumaz B, Feijs L, Hu J. A review of digital
cognitive behavioral therapy for insomnia (CBT-I Apps): are they designed for
engagement? Int J Environ Res Public Health. 2021;18(6):2929.
14. Koppel R, Kuziemsky C. Usability across health
information technology systems: searching for commonalities and consistency.
Stud Health Technol Inform. 2019;264:649-653.
15. Seppälä J, De Vita I, Jämsä T, et al. Mobile phone
and wearable sensor-based mHealth approaches for psychiatric disorders and
symptoms: systematic review. JMIR Ment Health. 2019;6(2):e9819.
16. Boonstra TW, Nicholas J, Wong QJ, et al. Using
mobile phone sensor technology for mental health research: integrated analysis
to identify hidden challenges and potential solutions. J Med Internet Res.
2018;20(7):e10131.
17. Saeb S, Zhang M, Karr CJ, et al. Mobile phone
sensor correlates of depressive symptom severity in daily-life behavior: an
exploratory study. J Med Internet Res. 2015;17(7):e175.
18. Lamichhane B, Ben-Zeev D, Campbell A, et al.
Patient-independent schizophrenia relapse prediction using mobile sensor based
daily behavioral rhythm changes. Paper presented at: Wireless Mobile
Communication and Healthcare: 9th EAI International Conference, MobiHealth
2020, Virtual Event; November 19, 2020. Proceedings 92021.
19. Tseng VW, Sano A, Ben-Zeev D, et al. Using
behavioral rhythms and multi-task learning to predict fine-grained symptoms of
schizophrenia. Sci Rep. 2020;10(1):15100.
20. Chen W. AmbianceCount: an objective social ambiance
measure from unconstrained day-long audio recordings. Master’s thesis. Rice
University; 2020.
21. Repple J, König A, de Lange SC, et al. Association
between genetic risk for type 2 diabetes and structural brain connectivity in
major depressive disorder. Biol Psychiatry Cogn Neurosci Neuroimaging.
2021;S2451-9022(21)00056-2.
22. Reus GZ, Carlessi AS, Silva RH, et al. Relationship
of oxidative stress as a link between diabetes mellitus and major depressive
disorder. Oxid Med Cell Longev. 2019;2019:8637970.
23. Sabharwal A, Fields SA, Hilliard ME, DeSalvo DJ.
Digital technologies to support behavior change: challenges and opportunities.
In: Diabetes Digital Health. 2020:37-50.
24. Avrahamy H, Shoval G, Hoshen M, et al. Association
between adherence to SSRI treatment and mortality among individuals with
metabolic syndrome components. Pharmacopsychiatry. Published online April 14,
2021.
25. Lee HM, Yang YC, Chen SF, et al. Risk of
hyperglycemic crisis episode in diabetic patients with depression: a nationwide
population-based cohort study. J Diabetes Complications. 2020;34(3):107509.
26. Brown KK, Kindratt TB, Boateng GO, Brannon GE.
Racial and ethnic disparities in healthcare rating, diabetes self-efficacy, and
diabetes management among non-pregnant women of childbearing age: does
socioeconomic status matter? J Racial Ethn Health Disparities. Published online
April 7, 2021.
27. McElfish PA, Rowland B, Scott AJ, et al. Examining
the relationship between physical activity and self-efficacy for exercise among
overweight and obese Marshallese adults. J Immigr Minor Health. Published
online April 10, 2021.
28. Pereira HV, Palmeira AL, Encantado J, et al. Systematic
review of psychological and behavioral correlates of recreational running.
Front Psychol. 2021;12:624783.